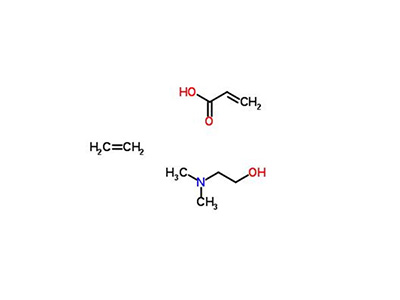
Anhydrous acetic acid
The melting point is 13.5°C, the boiling point is 140.9°C, and the density (20/4°C) is 1.0611g/cm3. The chemical nature is lively. It is easy to polymerize in air and can be reduced to propionic acid by hydrogenation. Addition with hydrogen chloride generates 2-chloropropionic acid. Used in the preparation of acrylic resins and other organic synthesis. It is made by oxidation of acrolein or hydrolysis of acrylonitrile, or made by carbon monoxide, acetylene and water under the action of nickel catalyst.
The melting point is 13.5°C, the boiling point is 140.9°C, and the density (20/4°C) is 1.0611g/cm3. The chemical nature is lively. It is easy to polymerize in air and can be reduced to propionic acid by hydrogenation. Addition with hydrogen chloride generates 2-chloropropionic acid. Used in the preparation of acrylic resins and other organic synthesis. It is made by oxidation of acrolein or hydrolysis of acrylonitrile, or made by carbon monoxide, acetylene and water under the action of nickel catalyst.
Colorless liquid, pungent odor, corrosive, and strong acidity. Soluble in water, ethanol and ether, but also soluble in benzene, acetone, chloroform, etc.