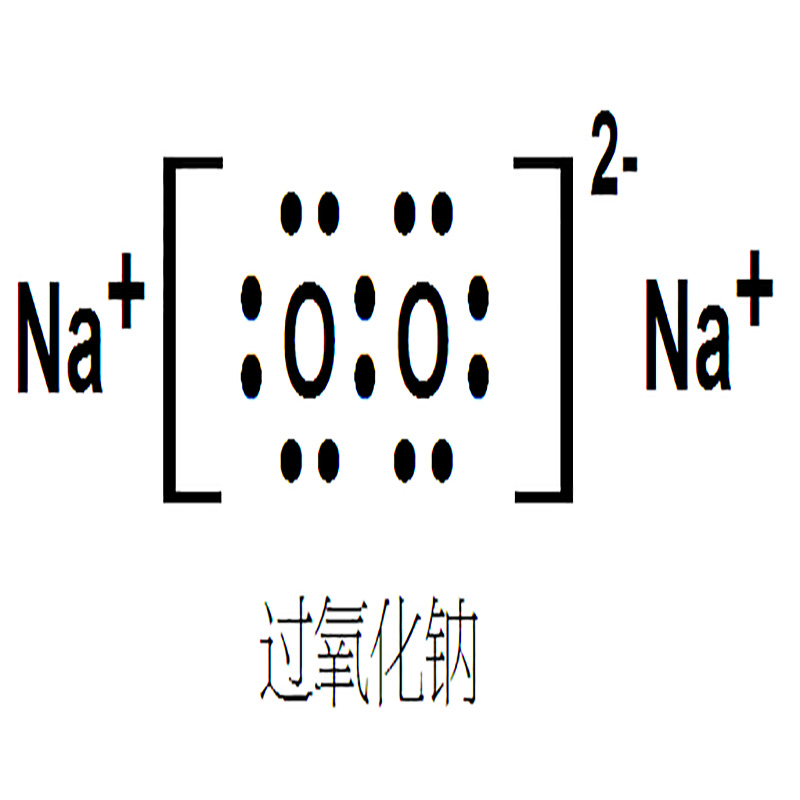
Sodium Peroxide
Differentiate when heated to 460℃. It is soluble in cold water, differentiated in hot water, and differentiated in ethanol or ammonia, soluble in dilute acid, but insoluble in alkaline solution. Quickly absorb moisture and carbon dioxide in the air. Contact with organic matter can cause incineration or explosion, so it should be kept tightly closed.
The octahydrate is a white hexagonal crystal, which differentiates when heated to 30°C, soluble in water, soluble in acid, and insoluble in alkali. It is obtained by reacting sodium in an aluminum drum heated to 300°C with air to remove carbon dioxide. Used for bleaching animal and plant fibers, feathers, animal bones, ivory, etc., as a printing and dyeing agent for fabrics, carbon dioxide absorbents in the air, submarine ventilation agents, chemical reagents, oxidants and analytical reagents
resolve resolution:
1. Metal sodium oxidation method: preheat molten sodium metal to above 180°C, spray a high-speed compressed air stream with a pressure of 39.2-44.1Pa into the oxidation furnace, and oxidize and incinerate the sodium at 300-350°C to generate peroxide Sodium is gradually cooled to below 100°C by free sedimentation to obtain sodium peroxide products.
2. Available metal sodium oxidation method. The preparation of Na2O2 is divided into two steps. First, metallic sodium is oxidized to sodium oxide in an atmosphere with oxygen content lower than air, and then sodium oxide is further oxidized to Na2O2 by heating at 200-350°C in an atmosphere with oxygen content higher than air. To complete the oxidation, the Na2O should be fully crushed before the second oxidation. However, moisture must be blocked.
3. Mix the 0℃ reagent saturated solution of sodium hydroxide and 42% hydrogen peroxide according to the theoretical proportion, and react. When the precipitate is no longer separated, filter with suction, and then wash with absolute ethanol and ether to obtain special Bright sodium peroxide octahydrate. It is also possible to dissolve industrial sodium peroxide in 4 times ice water, and the control temperature does not exceed 40°C. When the solution is cooled to 0°C, the crystals of sodium peroxide octahydrate are separated out, filtered and then suction filtered, and placed in the air without carbon dioxide. Sodium peroxide octahydrate is obtained by drying.