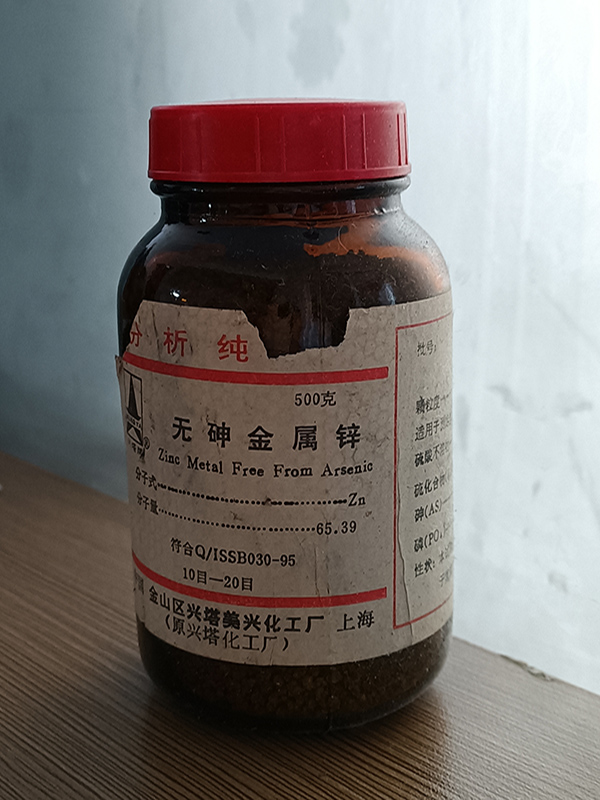
Arsenic-free Zinc
Product introduction: Strong reducing ability. It absorbs nitrogen in the air and oxygen when it is humid, and can burn when exposed to water. When its dust and air are mixed to a certain proportion, it will cause combustion or explosion.
Usage: Determination of arsenic, copper, lead, molybdenum and nitrate, soil and pesticide analysis, reducing agent, hydrogen production, zinc amalgam, etc. Used as a reducing agent or to replace certain metals and rare elements, dyes, medicines, etc.
Melting point: 419.6℃
Boiling point: 907℃
Density: 7.133
Storage: sealed and stored
Remarks: (easy to make explosives)
Arsenic-free zinc is an important inorganic compound with many important properties and uses. The following is an introduction to the properties, uses, preparation methods and safety information of arsenic-free zinc:
nature:
1. Arsenic-free zinc is a white crystalline powder, odorless.
2. It is a non-toxic and non-flammable compound.
3. Arsenic-free zinc is insoluble in water, but can be dissolved in acidic solution.
use:
1. Arsenic-free zinc can be used as an active ingredient in fungicides and insecticides and is used in agriculture to protect crops from fungi and insects.
2. It is also an important raw material for the manufacture of rubber, coatings, plastics and dyes.
3. Arsenic-free zinc is also widely used in electronics, batteries and optics.
Preparation method:
1. The most common method of preparing arsenic-free zinc is to react inorganic zinc salts (such as zinc chloride) with inorganic arsenic compounds (such as arsine).
2. This reaction produces zinc arsenide as an intermediate product, which is then further processed to convert it into arsenic-free zinc.
Security Information:
1. Arsenic-free zinc itself is non-toxic, but you need to pay attention to safe handling during production and use to avoid inhalation, contact with the skin or entering the esophagus.
2. When storing and handling arsenic-free zinc, care should be taken to avoid contact with acidic substances to avoid the generation of harmful gases.
3. If you accidentally come into contact with or swallow arsenic-free zinc, seek medical help immediately.