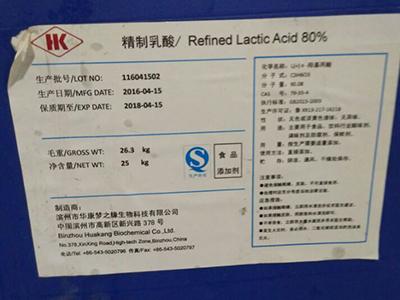
Chemical Products
Physical and chemical properties:
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
Physical and chemical properties:
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
Contact now
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
Contact now
Generally speaking, when the energy of the arrangement cannot be satisfied with oxygen breathing, it is arranged that the oxygen that cannot be obtained may not satisfy the quick treatment of oxygen, the concentration of lactic acid will rise. In this case, the acetone dehydrogenase cannot be converted to acetyl coenzyme a, pyruvate start-up. In this case, if the lactate dehydrogenase does not reduce the reduction of pyruvic acid as lactic acid, the composition of triphosphate is inhibited.
Generally speaking, when the energy of the arrangement cannot be satisfied with oxygen breathing, it is arranged that the oxygen that cannot be obtained may not satisfy the quick treatment of oxygen, the concentration of lactic acid will rise. In this case, the acetone dehydrogenase cannot be converted to acetyl coenzyme a, pyruvate start-up. In this case, if the lactate dehydrogenase does not reduce the reduction of pyruvic acid as lactic acid, the composition of triphosphate is inhibited.
Contact now
The lactate dehydrogenase is converted to a left lactic acid during the fermentation process. Lactic acids are constantly occurring in general metabolism and motion, but its concentration generally does not rise. Only in the lactic acid occurs, lactic acid cannot be transported in time to increase. The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
The lactate dehydrogenase is converted to a left lactic acid during the fermentation process. Lactic acids are constantly occurring in general metabolism and motion, but its concentration generally does not rise. Only in the lactic acid occurs, lactic acid cannot be transported in time to increase. The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
Contact now
Dissolving function: soluble in water and some solvents, such as ethanol/water, propanol/water in appropriate proportions. The aqueous solution has surface activity. High transparency and stable function. Different standard products have different gel temperatures and solubility changes with viscosity. The lower the viscosity, the greater the solubility. Different standards of HPMC have different functions. The dissolution of HPMC in water is not affected by pH.
Dissolving function: soluble in water and some solvents, such as ethanol/water, propanol/water in appropriate proportions. The aqueous solution has surface activity. High transparency and stable function. Different standard products have different gel temperatures and solubility changes with viscosity. The lower the viscosity, the greater the solubility. Different standards of HPMC have different functions. The dissolution of HPMC in water is not affected by pH.
Contact now
Appearance and properties: white or almost white fibrous or granular powder
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Appearance and properties: white or almost white fibrous or granular powder
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Contact now
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Contact now
White oblique crystals, industrial products are white or light gray lumps or rods. Easily soluble in water, emits a lot of heat of dissolution when dissolved, soluble in ethanol, slightly soluble in ether. It is deliquescent and has strong water absorption.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
White oblique crystals, industrial products are white or light gray lumps or rods. Easily soluble in water, emits a lot of heat of dissolution when dissolved, soluble in ethanol, slightly soluble in ether. It is deliquescent and has strong water absorption.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Contact now
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Contact now
Properties: White or slightly yellow scaly crystals with unique fragrance.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Properties: White or slightly yellow scaly crystals with unique fragrance.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Contact now
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Biphenyl is found in coal tar, crude oil and natural gas. The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Biphenyl is found in coal tar, crude oil and natural gas. The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Contact now
In fact, any kind of antioxidant can not fully meet these conditions. Therefore, in actual application, it is often based on the variety, use and processing method of engineering plastics, using the strengths of various additives and cooperating to produce synergistic effects.
In fact, any kind of antioxidant can not fully meet these conditions. Therefore, in actual application, it is often based on the variety, use and processing method of engineering plastics, using the strengths of various additives and cooperating to produce synergistic effects.
Contact now
Physical and chemical properties:
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
Contact Now
(1) Melting point (° C): - 2
(2) Boiling point (° C): 158 (no water)
(3) Relative density (water = 1): 1.46
(4) Relative density (air = 1): no material
(5) Solubility: dissolved in water, alcohol, ether, insoluble in benzene, petroleum ether
(6) Stability: unstable, protected from light
(7) Preventing the conditions of touch (taboo): strong acid, strong alkali, acyl chloride, alcohol, amine, flammable or combustible materials.
The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
Contact Now
Generally speaking, when the energy of the arrangement cannot be satisfied with oxygen breathing, it is arranged that the oxygen that cannot be obtained may not satisfy the quick treatment of oxygen, the concentration of lactic acid will rise. In this case, the acetone dehydrogenase cannot be converted to acetyl coenzyme a, pyruvate start-up. In this case, if the lactate dehydrogenase does not reduce the reduction of pyruvic acid as lactic acid, the composition of triphosphate is inhibited.
Contact Now
The lactate dehydrogenase is converted to a left lactic acid during the fermentation process. Lactic acids are constantly occurring in general metabolism and motion, but its concentration generally does not rise. Only in the lactic acid occurs, lactic acid cannot be transported in time to increase. The lactic acid transport speed is affected by a series of factors, and includes a monocarboxy-dient, lactate dehydrogenase concentration and isomer form, and the oxidative capacity arranged. Generally, the concentration of lactic acid in the blood is from 1-2 mmol / L during non-exercise, and can rise to 20 mmol / L during intense exercise.
Contact Now
Dissolving function: soluble in water and some solvents, such as ethanol/water, propanol/water in appropriate proportions. The aqueous solution has surface activity. High transparency and stable function. Different standard products have different gel temperatures and solubility changes with viscosity. The lower the viscosity, the greater the solubility. Different standards of HPMC have different functions. The dissolution of HPMC in water is not affected by pH.
Contact Now
Appearance and properties: white or almost white fibrous or granular powder
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Contact Now
Density: 1.39 g/cm3
Solubility: almost insoluble in absolute ethanol, ether, acetone; swelling in cold water into a clear or slightly dirty colloidal solution
Stability: The solid is flammable and incompatible with strong oxidants.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Contact Now
White oblique crystals, industrial products are white or light gray lumps or rods. Easily soluble in water, emits a lot of heat of dissolution when dissolved, soluble in ethanol, slightly soluble in ether. It is deliquescent and has strong water absorption.
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Contact Now
Potassium hydroxide is strongly alkaline and corrosive, and its properties are similar to sodium hydroxide, which can cause burns. It can absorb water and dissolve in the air, and absorb carbon dioxide to gradually become potassium carbonate. The pH of the 0.1 mol/L solution is 13.5.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Contact Now
Properties: White or slightly yellow scaly crystals with unique fragrance.
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Contact Now
Solubility: Insoluble in water, acid and alkali, but soluble in organic solvents such as alcohol, ether and benzene.
Biphenyl is found in coal tar, crude oil and natural gas. The preparation methods of biphenyl include the chemical composition method of making biphenyl through benzene pyrolysis and the separate extraction method of making biphenyl through various coal tar fractions. The mass fraction of biphenyl in coal tar is 0.20%-0.40%, and coal tar extraction method and chemical composition method coexist.
Contact Now
In fact, any kind of antioxidant can not fully meet these conditions. Therefore, in actual application, it is often based on the variety, use and processing method of engineering plastics, using the strengths of various additives and cooperating to produce synergistic effects.
Contact Now